FRANKENSTEIN CELLS?
 (Xinhua) — A team of University of Washington synthetic biology researchers have demonstrated a new method for digital information processing in living cells, analogous to the logic gates used in electric circuits.
(Xinhua) — A team of University of Washington synthetic biology researchers have demonstrated a new method for digital information processing in living cells, analogous to the logic gates used in electric circuits.
Reported in the latest issue of Nature Communications, the researchers built a set of synthetic genes that function in cells like Negated OR gates, or NOR gates, commonly used in electronics, which each take two inputs and only pass on a positive signal if both inputs are negative.
NOR gates are functionally complete, meaning one can assemble them in different arrangements to make any kind of information processing circuit.
In the microchips that run electronic computers, information processing capabilities reduce data to zeros and ones. In living cells, where constantly process information is taking place to keep track of the changing world around them and arrive at an appropriate response, deoxyribonucleic acid (DNA), proteins, lipids and sugars are arranged in complex and compartmentalized structures, as evolution has arrived at a mode of information processing at the cellular level.
The potential of cells as living computers that can respond to disease, efficiently produce biofuels or develop plant-based chemicals is what researchers have been working on and searching for.
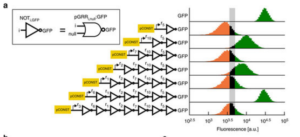
The UW researchers built their circuits, the largest ever published to date in eurkaryotic cells, using DNA instead of silicon and solder, and inside yeast cells instead of at an electronics workbench. Eurkaryotic cells, like human cells, contain a nucleus and other structures that enable complex behaviors.
Each cellular NOR gate consists of a gene with three programmable stretches of DNA — two to act as inputs, and one to be the output. The researchers then took advantage of a relatively new technology known as clustered regularly interspaced short palindromic repeats associated protein 9, or CRISPR-Cas9, to target those specific DNA sequences inside a cell.
The Cas9 protein acts like a molecular gatekeeper in the circuit, sitting on the DNA and determining if a particular gate will be active or not.
If a gate is active, it expresses a signal that directs the Cas9 to deactivate another gate within the circuit, enabling the researchers to “wire” together the gates to create logical programs in the cell.
What sets the new study apart from previous work, the researchers noted, is the scale and complexity of the circuits successfully assembled — which included up to seven NOR gates assembled in series or parallel.
At this size, circuits can begin to execute useful behaviors by taking in information from different environmental sensors and performing calculations to decide on the correct response. Imagined applications include engineered immune cells that can sense and respond to cancer markers or cellular biosensors that can easily diagnose infectious disease in patient tissue.
Cells could potentially be reprogrammed to undergo new developmental pathways, to regrow organs or to develop entirely new ones. In such developing tissues, cells have to make complex digital decisions about what genes to express and when, and the new technology could be used to control that process.
“While implementing simple programs in cells will never rival the speed or accuracy of computation in silicon, genetic programs can interact with the cell’s environment directly,” senior author and UW electrical engineering professor Eric Klavins, was quoted as saying in a news release. “For example, reprogrammed cells in a patient could make targeted, therapeutic decisions in the most relevant tissues, obviating the need for complex diagnostics and broad spectrum approaches to treatment.”